

Based on this trend, what do you estimate the density of silicon to be? The density of tin is about 7,28 g/cm3 and the density of Pb 11.34 g/cm3. Silicon (Si), tin (Sn), and lead (Ph) are all in the same group. We were able to figure this out using the information we already know, along with the knowledge we gained from this lab.Ĥ. As you move across the period (row) the elements also get less reactive. In general, is there a relationship between the locations of metals on the Periodic Table and their relative activity? Explain why.Īs you move down a group (column) the elements get less reactive. That being said, I also thought about how calcium did at least something, while tin did absolutely nothing in HCL, meaning it probably won't do anything in water.ģ. I took into account that water is pretty much neutral, and nothing major will happen to any of these elements in water. Use data from your lab to support your answer. List the four metals from most reactive to lease reactive. Because of the difference, they will react differently.Ģ. Magnesium is a solid while calcium is a powder. What might be a reason for the difference in behavior between magnesium and calcium when placed in water? Record data and observations in the table below.ġ. Add enough water to cover the sample (use a disposable pipette).Ĥ.
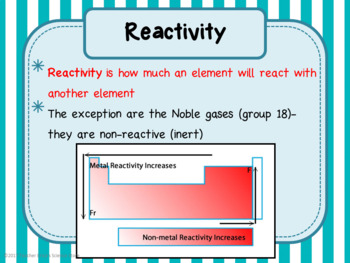
Get your samples from Charlene and put them in the labeled test tubes.ģ. Gather your supplies and label the test tubes.Ģ. Part one - trends in properties within groupsġ. What element is in Group three and Period three?Īluminum is in both group three and period three. Group one are the alkali metals, group two are the alkali earth metals.Ĥ. What are the names of two metal families? The metals are located to the left of the metalloid boundary.ģ. In general, where are the metals located on the periodic table? Families are specific groups on the periodic table.Ģ. What are groups? What are periods? What are families? To explore the reactivity trends of metals in groups and periods of the periodic table.ġ.


 0 kommentar(er)
0 kommentar(er)
